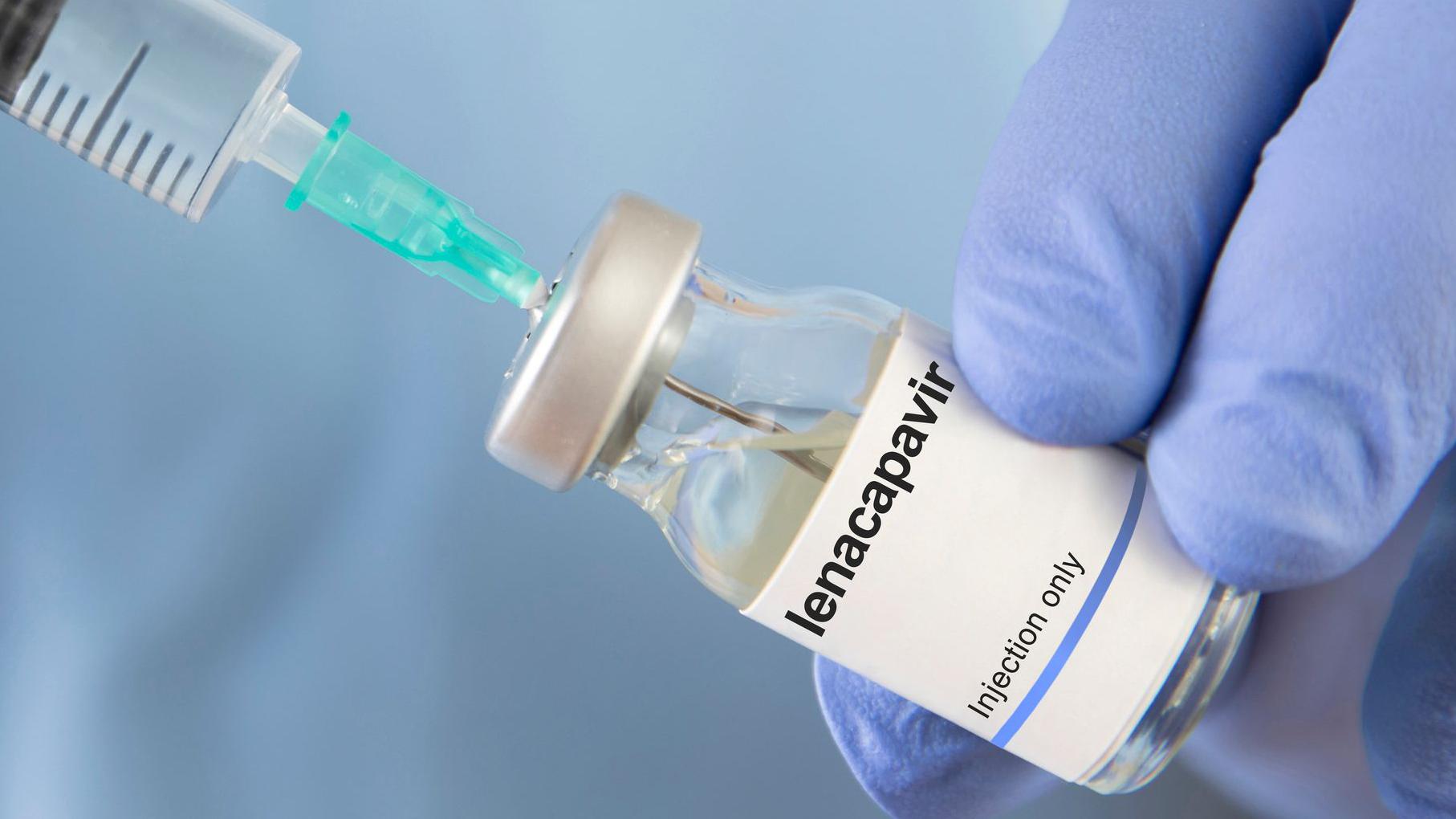
Uganda has made history in the battle against HIV/AIDS by granting approval to lenacapavir, a groundbreaking twice-yearly injectable drug that delivers six full months of protection against the virus in a single dose.
Announced by the National Drug Authority as a true “game-changer,” this long-acting pre-exposure prophylaxis (PrEP) offers a powerful alternative to daily oral pills, especially for those who face stigma, inconsistent healthcare access, or difficulty maintaining strict regimens. With Uganda’s adult HIV prevalence standing at approximately 5.4%, the arrival of lenacapavir strengthens the country’s resolve to help meet the global goal of ending AIDS as a public health threat by 2030.
Developed by Gilead Sciences, lenacapavir earned World Health Organization endorsement in July 2025 and prequalification in October, clearing the path for accelerated adoption across lower-resource regions. Uganda now stands proudly alongside South Africa, Zimbabwe, and Zambia—the only African nations currently providing this advanced prevention option to their citizens. Additional countries are advancing through the WHO’s streamlined registration process to broaden access further.
Daily oral PrEP has saved countless lives, but real-world adherence challenges have limited its impact. Lenacapavir sidesteps those hurdles entirely: just two injections annually provide near-complete protection, delivering both efficacy and discretion that can be transformative in communities where HIV-related stigma remains a daily reality.
Early evidence from clinical trials and initial rollouts elsewhere on the continent shows remarkable promise, particularly for young women, key populations, and rural residents with limited clinic options.
Just across the border, Kenya is gearing up for its own introduction of lenacapavir later in 2026. While excitement runs high, cost remains the primary obstacle. Pilot programs are expected to price each dose at around KSh 6,000, though partnerships with global health organizations and generic producers aim to lower the annual cost for two doses to between KSh 5,000 and KSh 5,200 in the near term. Longer-term targets, driven by increased generic availability, could bring individual doses down to KSh 2,500–2,600.
The need is undeniable. Kenya is home to roughly 1.33 million people living with HIV, with a national prevalence of 3.0%—rising to 4.1% among women versus 1.95% among men. In 2025 alone, the country recorded about 20,105 new infections and 21,009 AIDS-related deaths, underscoring the urgency of scalable, effective prevention tools.
Lenacapavir’s approval in Uganda—and its impending arrival in Kenya—marks a pivotal moment in decades of progress against one of the world’s most persistent epidemics. As manufacturing scales up and prices decline, this twice-yearly injection has the potential to protect millions more, dramatically curbing new infections and bringing the dream of an AIDS-free generation within reach.
With sustained commitment from governments, manufacturers, and international partners, lenacapavir could prove to be the turning point the world has been waiting for.