The Ministry of Health has launched a sweeping crackdown on the sale and misuse of Viagra following an exposé that laid bare alarming gaps in regulation, rampant abuse and weak oversight in Kenya’s pharmaceutical market.
Public Health Principal Secretary Mary Muthoni has reiterated that sildenafil citrate—popularly known as the “blue pill”—is not an over-the-counter drug, but a strictly prescription-only medicine. She warned that unsupervised use can trigger severe complications, including heart attack and stroke, particularly when combined with nitrates or certain blood-pressure and cardiac drugs.
“Sildenafil is legally restricted and intended only for specific medical conditions—erectile dysfunction and pulmonary arterial hypertension—under professional medical supervision,” Muthoni said.
Health officials say the urgency of the crackdown has been sharpened by reports linking sildenafil misuse to several high-profile deaths in Kenya, cases that sparked public concern and renewed scrutiny of illegal access to the drug. While investigations have underscored the complexity of causation, authorities note that widespread misuse by unqualified users—including minors—has become increasingly evident, fuelled by illegal retail, online sales and social media marketing.
Medical experts warn that adolescents and young adults face heightened risks due to lack of screening for underlying conditions and unsafe mixing with alcohol or other substances.
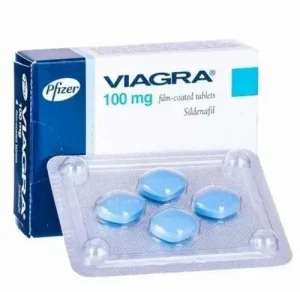
In response, the ministry has rolled out concrete actions to seal loopholes across the supply chain. These include mandatory sales reporting by wholesalers, routine audits and inspections of pharmacies to review prescription records, and expanded public awareness campaigns highlighting the dangers of self-medication.

The Pharmacy and Poisons Board (PPB) has reinforced the warning, cautioning manufacturers, importers, distributors, retailers and online sellers against dealing in unregistered, falsified or counterfeit medicines. The regulator stressed that supermarkets, cosmetic shops and general retail outlets are not authorized to stock or sell prescription-only medicines, and violations will attract prosecution, licence suspension or closure of premises.
Liability, PPB said, extends to superintendent pharmacists, managers, directors and owners of offending establishments.
Authorities are urging the public to help curb the menace by reporting suspicious medicines or adverse drug reactions via the *PPB online portal, 271# USSD code, the mPvERS mobile app, or official PPB contacts.
“You don’t need to be certain—just be suspicious,” the board advised.
As enforcement tightens, regulators insist the message is clear: illegal access to the “blue pill” will no longer be tolerated, and those endangering lives through unlawful trade and misuse will face decisive action.







Leave a Reply